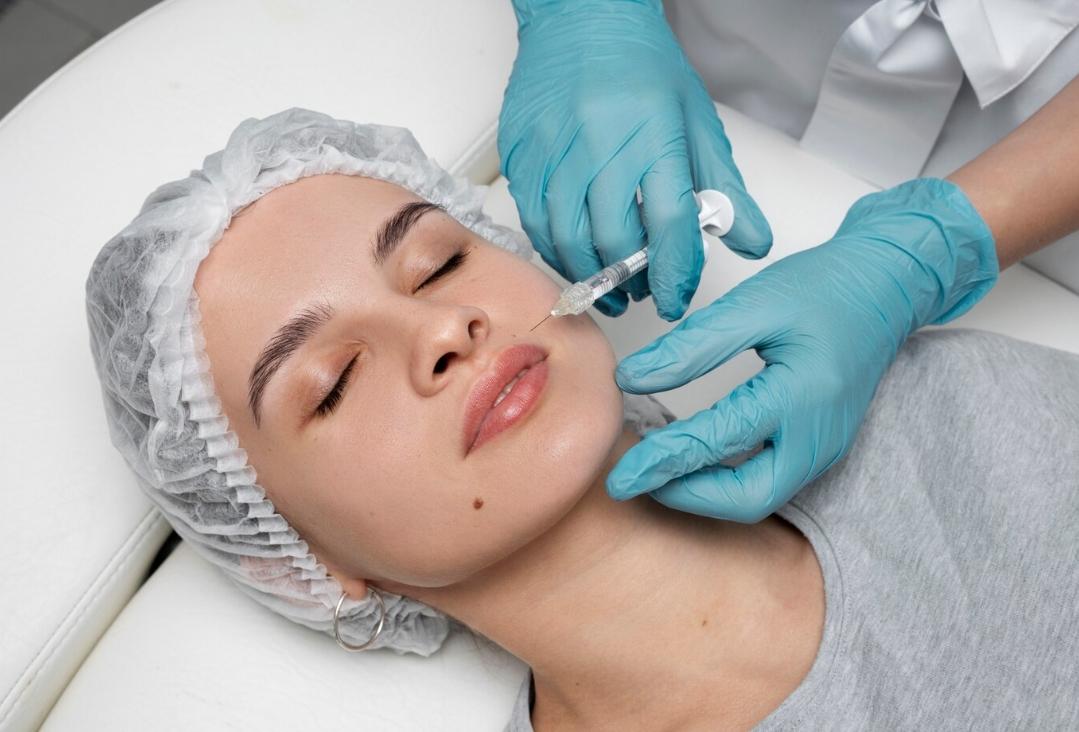
In order to empower the Pharmaceutical Grade Sodium Hyaluronate market and meet higher market demand, Topscience Biotech has built the first workshop for Sterile Grade Sodium Hyaluronate in China. The product has high safety and applicability, and can be applied to high-end medical aesthetic fillings and drug products where it can give full play to its functions of moisturizing, viscoelastic lubrication and so on.
Product Overview
"Sterile raw materials can be divided into two categories according to the production process: one is final sterilization raw materials produced by a final sterilization process, and the other is non-final sterilization raw materials part or all of which are produced by a sterile production process.
The non-final sterilized products are be sterilized by means of sterilization and filtration to ensure the product quality via strict sterile control. The sterile HA raw materials produced by the sterile production process can be used by downstream manufacturers of sterile preparations and sterile medical devices without further final sterilization procedures."
Product Advantage
Sterile grade sodium hyaluronate is produced using aseptic processing and is truly a sterile grade product that meets regulatory requirements. However, traditional sodium hyaluronate achieves sterility only for the purpose of meeting the requirements for sterility, which is called testing sterility. The production process does not comply with regulatory requirements.
Since Sodium Hyaluronate will degrade under high temperature conditions, this is its general property. For customers who need sterile raw materials, using STERILEHYA will no longer need to consider the risk of degradation caused by sterilization, which cut the cost of experiments and time.
STERILEHYA is sterile raw material, which can be used directly for the sterile medicinal products & sterile medical devices which cannot be sterilized in their final containers.
The 'sterile' sodium hyaluronate that currently exists on the market is only the sodium hyaluronate that has passed the sterility test. The sterile sodium hyaluronate launched by Topscience Biotech is produced by using the production process of sterilization filtration, which is the true meaning of sterile sodium hyaluronate. The sterile sodium hyaluronate belongs to the highest-end branch of pharmaceutical grade sodium hyaluronate. Topscience Biotech is the first enterprise in China to launch sterile sodium hyaluronate drug substance, and no other company in the industry has launched the same grade product at present. The sterile sodium hyaluronate produced by Topscience Biotech meet the terminal application standards in the downstream pharmaceutical field, which is a major technology innovation after pharmaceutical grade Sodium Hyaluronate.
——Quoted from 'White Paper of China Sterile Hyaluronic Acid'
Apply for White Paper
The 'White Paper of China Sterile Hyaluronic Acid' (hereinafter referred to as the 'Report') is jointly released by Frost & Sullivan and Topscience Biotech. The report focuses on four major chapters, including hyaluronic acid raw material industry, China's sterile hyaluronic acid raw materials, sterile hyaluronic acid terminal applications, and the competitive landscape of the sterile hyaluronic acid raw material market.